- New
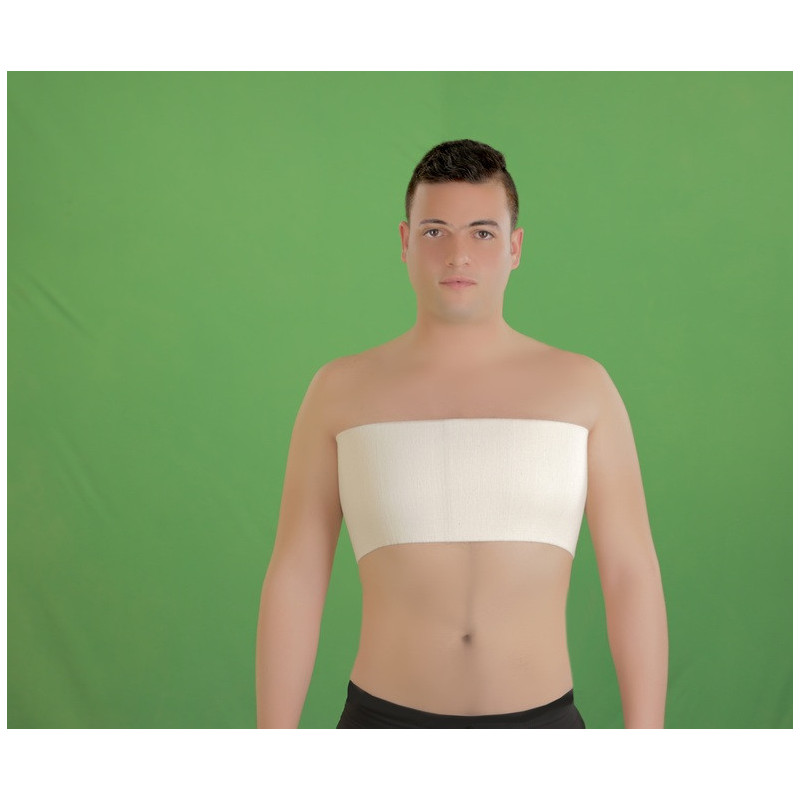

- Post-operative support (Open heart surgery)
- Fracture, fissure, strained or sprained ribs
- Size: S,M,L,XL,XXL,XXXL
Instructions For Use ( I F U )
- Trade Name : Care Chest Binders
كير تشست بيندرز - Arabic Name:
- Registered Trade Name Of the Manufacturer :
Healthy Health Care Products
- Registered Place of Business ( Address ) :
99 Al Sabak ST. – Meriland Heliopolis – Cairo – Egypt
- Where Applicable :
Home and Hospitals
Application :
directly applied around chest walls from behind to front and fasten the belt by Velcro anteriorly , It is preferably to be applied directly on the bare skin .
- How Determine the suitable size ( Sizing ) ? :
A) Male and child :
* Measure chest circumference from below axilla and on the nipples
* Use the sizing chart on the package to get the suitable size
B) Female :
* Measure chest circumference from below axilla and below the breasts .
* Use the sizing chart on the package to get the suitable size
- Lot or Serial Number :
MM / YY
- Sterility :
The Device is supplied unsterile
- Single use or reusable :
The device is reusable unless it is teared or become redundant .
- The duration of use : for 3 months to 6 months
- Invasive or non invasive :
The device is non invasive
The device not introduced into human body orifices
The device not absorbed by human skin or locally dispersed
- Intended Purpose :
Chest Binders are tightened around the Chest , to limit movements of thoracic cage , to enhance healing for bone , joints , muscles post operative or post inflammation due to trauma or diseases and also have a thermal effect .
- Indications :
* post operative ( open Chest operations )
* post traumatic ( ribs , costal joints and inter coastal muscles)
* Inflammation of periosteum , costal joints or intercostal muscles.
- Precautions :
* Be sure of right size
* Be sure of sanitary conditions ( dry & clean )
* Reusable for not more than 6 months
* The device must not be teared or redundant
- Contra indications :
* Inflamed Skin
* Teared or redundant device
- Device description:
* non-sterile, cotton 100% cloth with Velcro for fastening, applied by the patient himself or by nursing staff. Applied either post operative or post trauma to support anterior, lateral, and posterior chest walls (i.e. ribs and intercostal muscles). This is multiple use device and can undergo any type of sterilization
- Suggested Training for users
The device needs no training for users
- Performance Characteristics :
* Non invasive
* Already used non sterile but can be sterilized either by Ethylene Oxide or Gamma Radiation .
* Not rupture for proper strong tighten performance .
* Easy to use i . e easy to wear and easy to get off .
* Available sizes to suit all patients , both adults and children .
* Material in contact with the skin is cotton 100% ( the most safest material )
* Strong elastic cotton cloth .
* Wide Velcro ( soft ) for good tight adjusted fastening
- Device specification :
* Support the anterior, lateral and posterior chest walls ( including ribs and intercostal muscles )
* Allows but restrict complete free movements of chest walls .
* Elastic for cool , comfortable and extended use .
* Completely , washable and hygienic
- Special Facilities :
The device requires no , special facilities , special training or particular qualification of the user
- Device maintenance and calibration :
The device needs no , regular maintenance , no consumable components , and no necessary calibration or measurements
- Instructions for cleaning :
* Put the device in tape water with some detergent for a while
* Rinse the device è tape water to remove remnant for detergent
* Dry the device away from direct sunlight , in horizontal position
* No automatic washing machine
* No electric iron
- Other devices :
Not used together with other devices
- Circumstances in which the user should consult a health care professional :
Only if the device is teared , ruptured , redundant or dirt
- Any serious incident , occurred in relation to the device should be reported to :
1- The manufacturer
2- SFDA
- Date of issue : 04/2025